Abstract
Background: JAK-inhibitor (JAKi) therapy can improve MF-related symptom burden and quality of life (QOL) for patients with myelofibrosis (MF). Clinical benefit is variable with most patients experiencing failure of firstline (1L) JAKi therapy by 3 years and subsequent survival of 11-16 months. At our centre, hematopoetic stem cell transplant (HCT) is considered in patients after 1L JAKi failure who are fit for transplant. Many patients do not prefer HCT despite donor availability due to concerns of adverse impact on QOL and mortality. The approval of fedratinib and pacritinib as second-line (2L) JAKi and ongoing development of novel therapies provide new options for patients with JAKi failure. Data on clinical outcomes with or without HCT following 1L JAKi failure are limited, with prospective studies logistically challenging in a rare disease.
Aims: To compare the overall (OS) and transformation-free (TFS) survival following 1L JAKi failure in chronic-phase MF patients who receive HCT or best available non-transplant therapy (BAT).
Methods: We conducted a retrospective review of patients with MF from the Princess Margaret prospective MPN registry (NCT02760238) treated with 1L JAKi from 2012-2021. Patients were included in the study if they had failure of 1L JAKi therapy as defined by Canadian MPN Group criteria, and excluded if they were not fit for HCT due to: age >70 years, death ≤8 weeks of JAKi failure, accelerated/blast phase (AP/BP), active second cancer, prohibitive performance status or co-morbidities. Patients included in the BAT group received 2L JAKi, clinical trial novel agent (alone or in combination with JAKi), splenectomy, transfusion support, or other non-transplant therapy. Survivals were calculated from time of JAKi-failure to last follow-up, death (OS), or transformation to AP/BP (TFS).
Results: Of the 641 patients with MF in our database, 323 (50.4%) were treated with 1L JAKi and 88 considered HCT-eligible following JAKi failure. The 1L JAKi was ruxolitinib in 75 (85%) patients and momelotinib in 13 (15%). Patterns of JAKi failure were: suboptimal/loss of spleen response n=57 (65%), cytopenias n=27 (31%), non-hematologic toxicity n=4 (4%). From time of JAKi failure the median [range] follow-up was 21.0 [3-126] months.
Following 1L JAKi failure 41 (47%) patients underwent HCT; and 47 (53%) received BAT including 2L JAKi (fedratinib n=7, momelotinib n=6, ruxolitinib n=3, pacritinib n=3), investigational agent added to ruxolitinib (n=3), continuation of 1L JAKi (n=18), splenectomy (n=6), and splenic radiation (n=1). Two patients in the BAT group underwent salvage HCT following failure of subsequent treatment. In the HCT group 8 patients received bridging 2L treatment (fedratinib n=4, ruxolitinib n=2, splenectomy n=2).
At time of JAKi failure the HCT cohort was younger (median [range] 60[38-69]y vs. 65[49-70]y, P=0.002), had smaller palpable spleen size (10[0-26] vs. 17[0-35]cm, P<0.001), less frequent constitional symptoms (15% vs. 35%, P=0.03), and greater proportion of low/int-1 age-adjusted DIPSS (aaDIPSS 59% vs 28%, P=0.02). There was no observed significant differences in ECOG status, blood/BM blast counts, RBC transfusion needs, or pattern of failure.
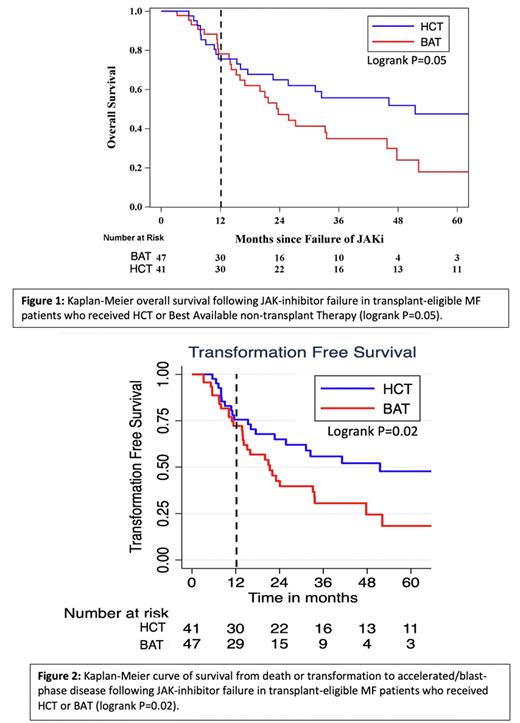
The median [95% confidence interval] OS(Fig 1) and TFS (Fig 2) were 51 [23-Not Reached] and 51 [23-89] months respectively in the HCT group; and 24 [16-46] and 21 [14-34] months in the BAT group. Survival rates in two arms were similar up to 12 months after JAKi failure (OS 76% vs 78%; TFS 76% vs. 72%), but began to diverge in favour of HCT at 24 months (OS 65% vs. 47%, TFS 65% vs. 43%) and 36 months (OS 56% vs. 35%, TFS 56% vs. 31%). Multivariable Cox proportional hazards model adjusted for age and aaDIPSS did not observe significant benefit for HCT (OS Hazard Ratio 0.68 [95%CI 0.37-1.27]; TFS 0.60 [0.32-1.1]).
At the last follow up, there were 21 (51%) and 28 (59%) deaths recorded in the HCT and BAT arms, respectively. The main causes of death were transplant-related complications (n=14) and relapsed/progressive disease (n=4) in the HCT cohort; and progression of MF with (n=11) or without (n=12) AP/BP transformation in the BAT arm. Despite a large cohort of JAKi-treated patients, the number of HCT-eligible patients is small and is the main limitation of this study.
Conclusion: Patients with MF and failure of first line JAKi managed with HCT or BAT have similar short-term outcomes, but trend towards better OS and TFS with HCT after 12 months.
Disclosures
Gupta:BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria; Roche: Other: Participation on a Data Safety or Advisory board; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal